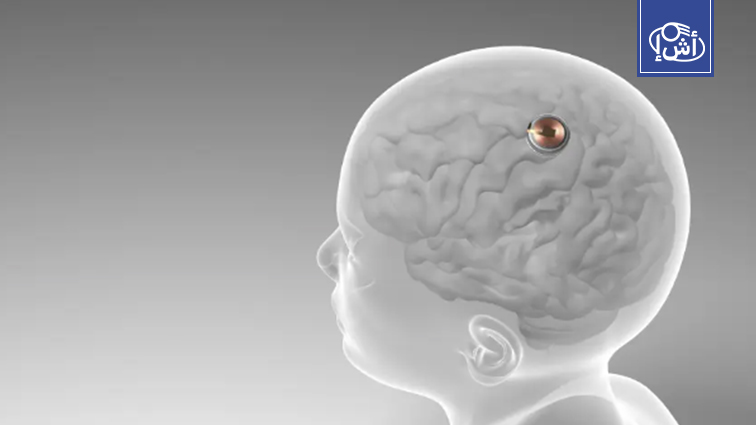
Neuralink, owned by billionaire Elon Musk, has announced the development of a new brain chip to treat neurological disorders and paralysis.
The company has received approval from the U.S. Food and Drug Administration to begin clinical trials in humans, after the company had an earlier trial that showed limited success in helping a quadriplegic patient control computers and phones with only ideas.
The previous segment suffered from some technical problems, such as the early disconnection of some fine wires, but the company’s recent modifications contributed to improving the functionality of the device.
Software updates implemented after the first round of trials have also helped restore many of the chip’s core functions, suggesting significant advances in brain-computer technology.
The Wall Street Journal quoted a source familiar with the matter as saying that Neuralink has successfully addressed some of the past flaws, opening the door to vast possibilities for this technology in the future.
In March, the company showed a video of brain-transplanted Noland Arbo playing chess alone using the implanted chip.
Neuralink is preparing to conduct larger-scale clinical trials to obtain the necessary approvals to commercialize the procedure, raising many hopes among patients around the world that vital functions lost due to neurological injuries or paralysis will be restored.
Neuralink was founded in July 2016 with the aim of developing computing interfaces that support the human brain, or what is known as brain-computer interfaces.
In January 2024, the company announced the completion of the first chip implant in the human brain, and on February 15, 2024, Elon Musk stated that the person whose brain was implanted the chips fully recovered and became able to control the computer mouse through thinking.
The launch of the latest version of the “Grok” robot causes widespread controversy due to offensive images